Current research at UC Davis
Diversity of Mitochondrial Respiration
1. Padavannil. A., Murari, A., Rhooms, S.-K., Owusu-Ansah, E., Letts, J.A., (2023) Resting mitochondrial complex I from Drosophila melanogaster adopts a helix-locked state. eLife
2. Zhou, L.,* Maldonado, M.*, Padavannil, A., Guo, F., Letts, J.A. (2022) Structures of Tetrahymena’s respiratory chain reveal the diversity of eukaryotic core metabolism. Science. abn.7747
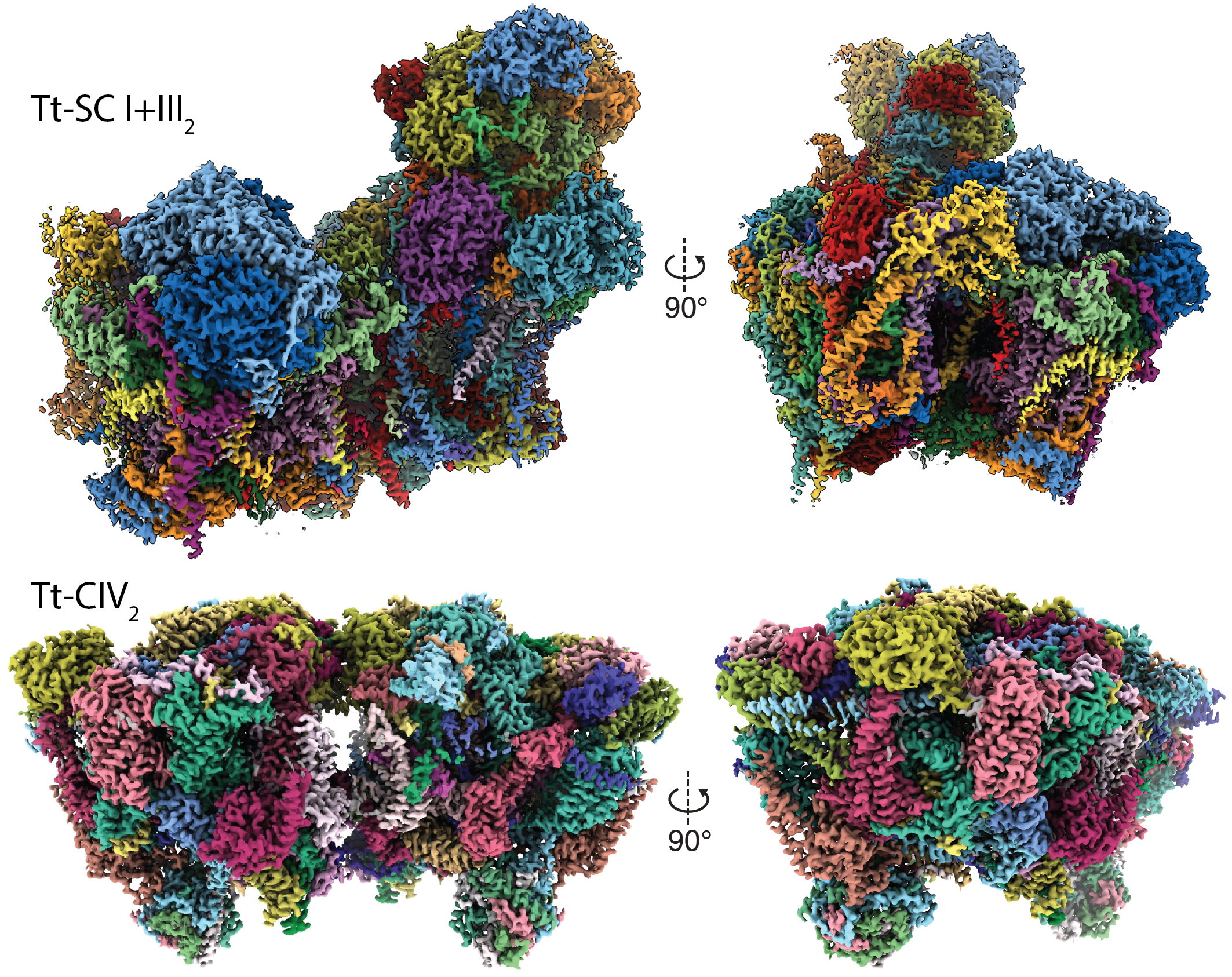
Plant Mitochondrial Respiration
Although plants are most famous for their ability to perform photosynthesis, mitochondrial respiration is still an essential process for plant energy production. Check out our recent paper and pre-print from the lab presenting the first structures of plant mitochondrial respiratory complexes!
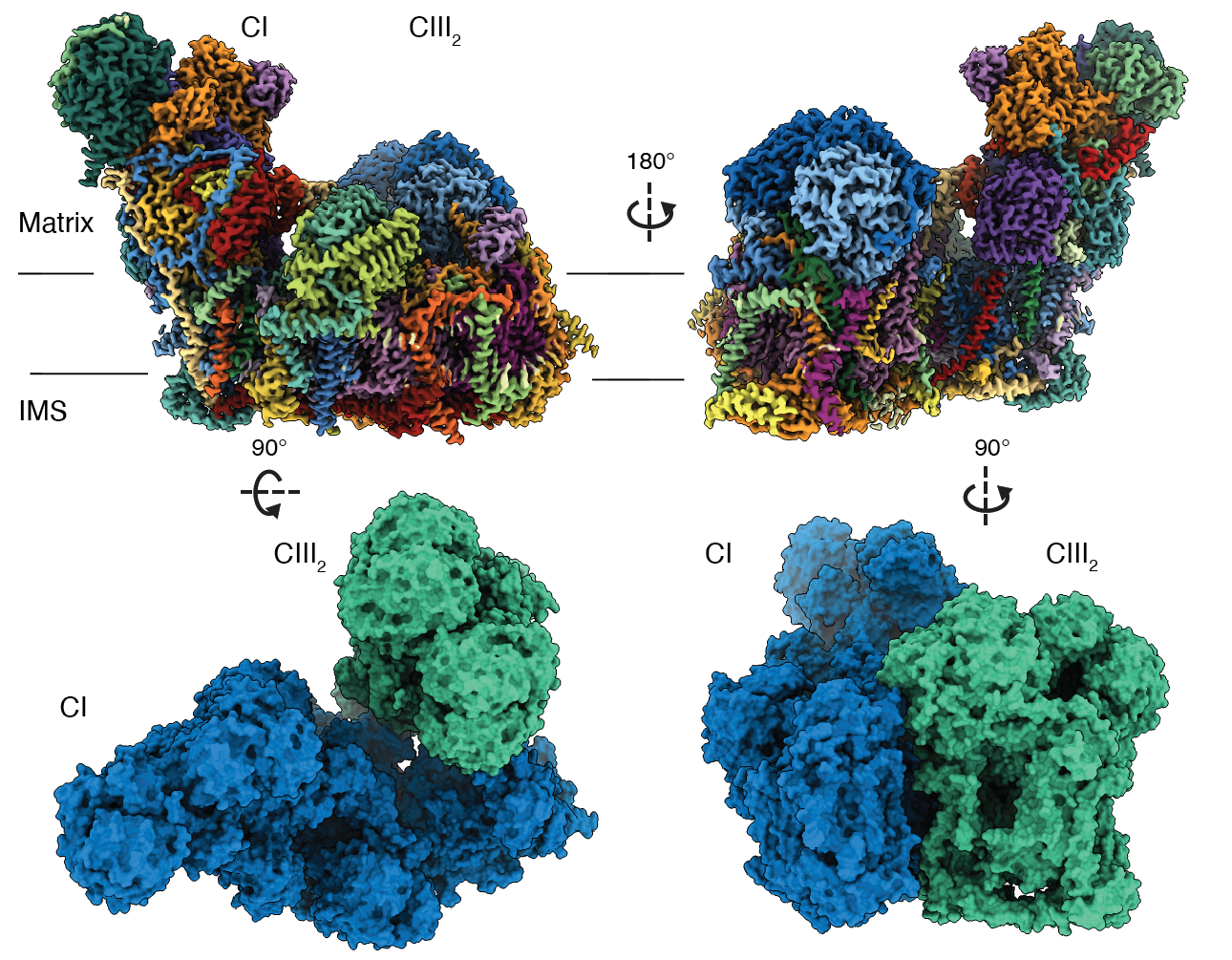
- Maldonado, M., Fan, Z., Abe, K.M., Letts, J.A. (2022) Plant specific features of respiratory supercomplex I+III2 from Vigna radiata. Nature Plants.
- Meyer, H.E., Letts, J.A., Maldonado, M. (2022) Structural insights into the assembly and the function of the plant oxidative phosphorylation system. New Phytologist; doi:10.1111/nph.18259
- Maldonado, M., Abe, K.M., Letts, J.A. (2022) A Structural Perspective on the RNA Editing of Plant Respiratory Complexes. J. Mol. Sci., 23, 684.
- Maldonado, M., Guo, F., Letts, J.A. (2021) Atomic structures of respiratory complex III2 , complex IV and supercomplex III2+IV from vascular plants. eLife; 10:e62047
- Maldonado, M., Padavannil, A., Zhou, L., Guo, F., Letts, J.A. (2020) Atomic structure of a mitochondrial complex I intermediate from vascular plants. eLIfe; 9:e56664
Check out the eLife Insight article regarding this work by Professor Hans-Peter Braun.
Please see our current research projects here.
Mammalian Mitochondrial Respiration
- Padavannil, A., Ayala-Hernandez, M.G., Castellanos-Silva, E.A., Letts, J.A. (2022) The Mysterious Multitude: Structural Perspective on the Accessory Subunits of Respiratory Complex I. Front. Mol. Biosci.; 8 1252
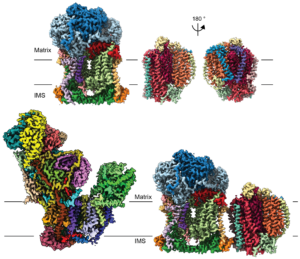
Dr Letts’ previous work
Mitochondrial Electron Transport
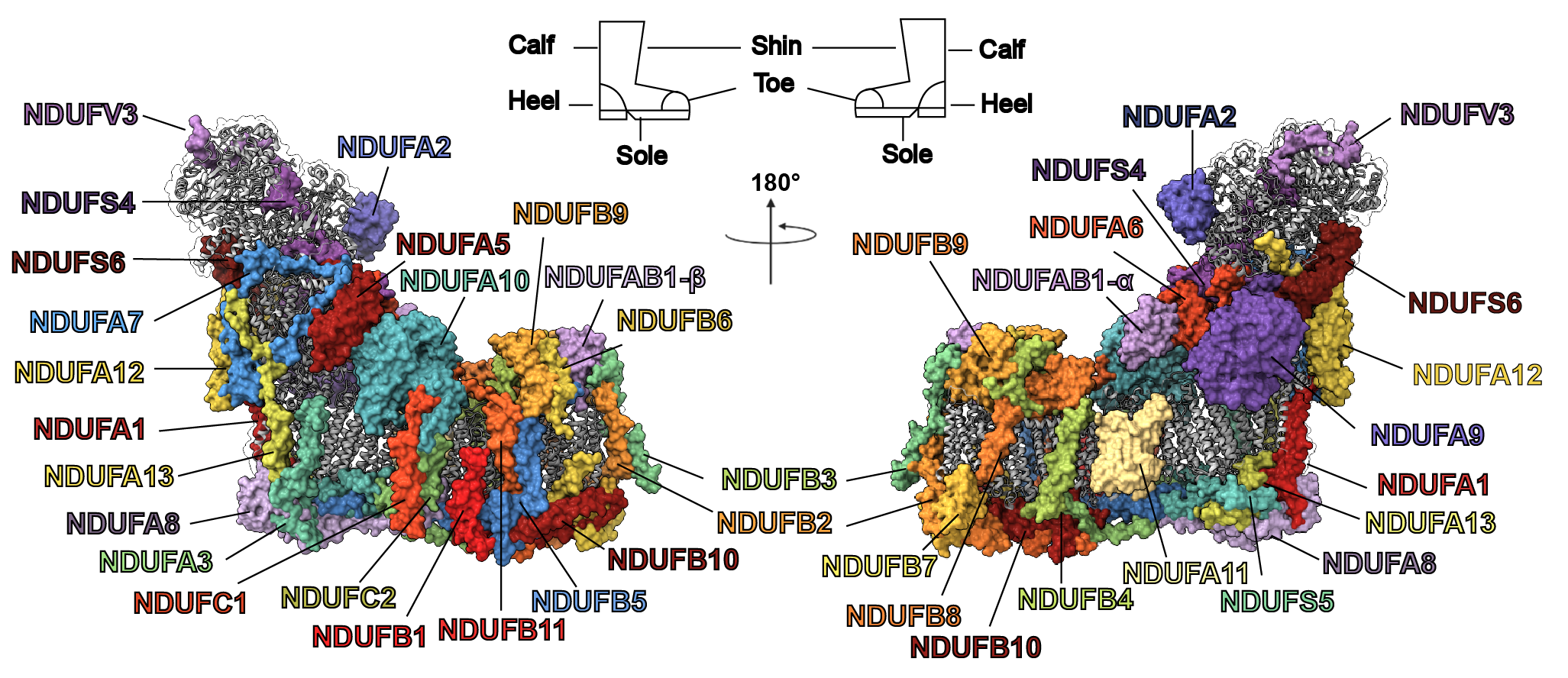
As a postdoctoral fellow in Dr Leonid Sazanov‘s lab, I studied the structure and function of the large membrane protein complexes and supercomplexes of the mammalian mitochondrial electron transport chain. The main structural approach we implemented was electron cryo-microscopy (cryo-EM). Specifically, I developed a novel protocol for the purification of complex I from sheep-heart mitochondria that results in a highly stable and active complex (Letts et al., JBC 2016). Using this protocol, we solved the first atomic structure of complex I by single-particle cryo-EM (Fiedorczuk et al., Nature 2016). In parallel, I solved the architecture of the respiratory supercomplex formed by complex I, complex III2 and complex IV (known as the respirasome) in two states, as well as the architecture the supercomplex formed between complex I and complex III2 alone (Letts et al., Nature 2016). The impact of my work is illustrated by the inclusion of our structure in the first figure of the Scientific Description of the 2017 Nobel Prize in Chemistry as an example of how the new developments in cryo-EM have been used to make fundamental discoveries.
-
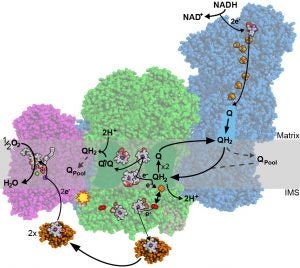
The respirasome. Letts, J.A., Fiedorczuk, K., Degliesposti, G., Skehel, M., Sazanov, L.A. (2019) Structures of Respiratory Supercomplex I+III2 Reveal Functional and Structural Crosstalk. Molecular Cell 74; 1-16.
- Letts, J.A., Sazanov, L.A. (2017) Clarifying the Supercomplex: The higher-order organization of the mitochondrial electron transport chain. Nature Structural and Molecular Biology 24; 800-808. Review article.
- Letts, J.A., Degliesposti, G., Fiedorczuk, K., Skehel, M., Sazanov, L.A. (2016) Purification of Ovine Respiratory Complex I Results in a Highly Active and Stable Preparation. Journal of Biological Chemistry 291; 24657-24675.
- Fiedorczuk, K., Letts, J.A., Degliesposti, G., Kaszuba, K., Skehel, M., Sazanov, L.A. (2016) Atomic structure of the entire mammalian mitochondrial complex I. Nature 538; 406-410. Letter, Oct 20.
- Letts, J.A., Fiedorczuk, K., Sazanov, L.A. (2016) The architecture of respiratory supercomplexes. Nature 537; 644-648. Article, Sept 29.
- Letts, J.A., Sazanov, L. A. (2015) Gaining Mass: the structure of respiratory complex I – from bacterial towards mitochondrial versions. Current Opinion in Structural Biology 33; 135-145. Review article.
This work was supported by a FEBS Long-Term Fellowship and a Marie Skłodowska-Curie Individual Fellowship (MSC-IF).
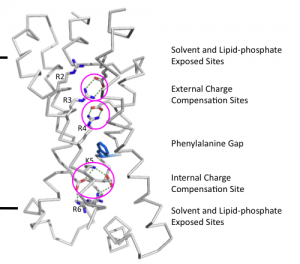
Voltage-gated H+ channel
My doctoral research at Rockefeller University under Dr Roderick MacKinnon focused on structural and functional studies of the human voltage-gated proton channel (hHV1). By expressing the channel in HEK293 cells and performing site specific cross-linking in the membrane, I (together with a post-doc collaborator Dr Seok-Yong Lee) demonstrated that this channel is a functional dimer (Lee, Letts and MacKinnon, PNAS 2008). Furthermore, through expression of hHV1 in Pichia pastoris, purification and reconstitution, we demonstrated that the channel was able to conduct protons independent of any cellular factors (Lee, Letts and MacKinnon, JMB 2009). Given that raising antibodies against the membrane portion of the channel for antibody mediated crystallization proved intractable, a chimeric approach was taken in which I spliced a known antibody epitope of a homologue into the voltage-sensor domain. In this way, I was able to obtain diffraction-quality crystals of an hHv1-Fab complex and solve its structure. However, the maps revealed that the channel in the crystal was in a non-physiological, partially unfolded state. Thus, I decided to adopt a different approach and, in collaboration with a post-doc (Dr Joel Butterwick), attempted to solve the structure of the hHv1 voltage-sensor domain using NMR. Although we were able to clearly define the secondary structure of the protein, we again found that the protein became partially unstructured in detergent. As each construct was functional upon reconstitution, the main lesson from these studies was the importance of the membrane bilayer for the stabilization of voltage-gated channels and the importance of structure validation using biochemical and biophysical approaches.
My thesis was officially accepted and published on my graduation day of June 6th 2014.
- Letts, J.A. (2014) Functional and Structural Studies of the Human Voltage-Gated Proton Channel. The Rockefeller University DSPACE: https://digitalcommons.rockefeller.edu/student_theses_and_dissertations/177/. PhD Thesis.
- Lee, S.Y.*, Letts, J.A.*, MacKinnon, R. (2009) Functional reconstitution of purified human Hv1 H+ channels. Journal of Molecular Biology 387; 1055-1060. (*equal first author)
- Lee, S.Y., Letts, J.A., MacKinnon, R. (2008) Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proceedings of the National Academy of Sciences 105; 7692-7695.
My PhD research was supported by the Post Graduate Scholarship M (2007 to 2009) and the Post Graduate Scholarship D (2009 to 2011) from the Natural Science and Engineering Council of Canada.
BLAST FROM THE PAST: Watch Dr. Letts defend his PhD thesis! This video contains the public lecture portion of Dr. Letts’ thesis defense (a.k.a. viva) from September 26th, 2013. Introduction by Dr. Roderick MacKinnon.
Human ABO(H) Glycosyltransferases and carbohydrate recognizing antibodies
I became involved with research early on as an undergraduate at the University of Victoria through a work-study program. This program allowed me in my second year to work part time in the laboratory of Dr. Stephen Evans where I began work in X-ray crystallography studying the structures of the human ABO(H) glycosyltransferase enzymes and carbohydrate binding antibodies. Through the co-operative education program, which interspersed terms of lectures with terms of lab work, I was able to work full-time with Dr. Evans each other term and part-time during the lecture terms (the academic year was split into three equal terms). Working with Dr Evans is where I initially learned the methods of molecular biology, protein purification, crystallization, and X-ray crystallographic structure determination. Using molecular replacement, I solved the structures of glycosyltransferase enzymes in complex with several different substrate analogues as well as several mutant enzymes.
The work on glycosyltransferases in Dr. Evans’ lab was largely performed in collaboration with Dr. Monica Palcic at the Carlsberg Research Center in Copenhagen Denmark. The collaboration between Dr. Evans and Dr. Palcic was put on hiatus for several years and then recently started up again. This is why some of my work from my undergraduate studies is only now being published.
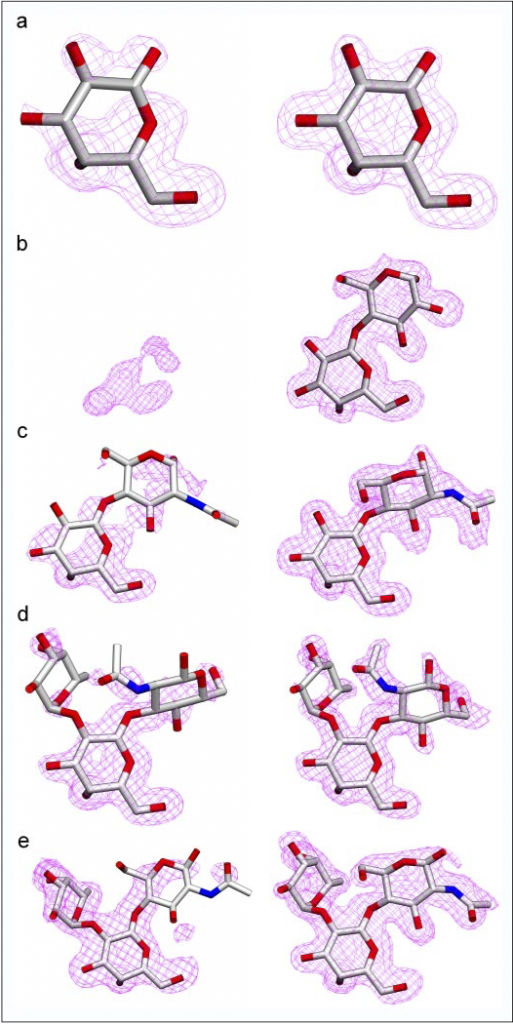
- Gagnon, S.M.L., Legg, M.S.G., Polakowski, R., Letts, J.A., Persson, M., Lin, S., Zheng, R.B., Rempel, B., Schuman, B., Haji-Ghassemi, O., Borisova, S.N., Palcic, M.M., Evans, S.V. (2018) Conserved residues Arg188 and Asp302 are critical for active site organization and catalysis in human ABO (H) blood group A and B glycosyltransferases. Glycobiology 28; 624-636.
- Gagnon, S.M.L., Legg, M.S.G., Sindhuwinata, N., Letts, J.A., Johal, A.R., Schuman, B., Borisova, S.N., Palcic, M.M., Peters, T., Evans, S.V. (2017) High-resolution crystal structures and STD NMR mapping of human ABO(H) blood group glycosyltransferases in complex with trisaccharide reaction products suggest a molecular basis for product release. Glycobiology 27; 966-977.
- Blackler, R.J., Gagnon, S.M., Polakowski, R., Rose, N.L., Zheng, R.B., Letts, J.A., Johal, A.R., Schuman, B., Borisova, S.N., Palcic, M.M., Evans, S.V. (2017) Glycosyltransfer in mutants of putative catalytic residue Glu303 of the human ABO(H) A and B blood group glycosyltransferases GTA and GTB proceeds through a labile active site. Glycobiology 27; 370-380.
- Johal, A.R., Jarrell, H.C., Letts, J.A., Kheiu, N.H., Landry, R.C., Jachymek, W., Yang, Q., Jennings, H.J., Brisson, J-R., Evans, S.V. (2013) The antigen-binding site of an N-propionylated polysialic acid-specific antibody protective against group B meningococci is consistent wit extended epitopes. Glycobiology 23; 946-954.
- Alfaro, J.A., Zheng, R.B., Persson, M., Letts, J.A., Polakowski, R., Bai, Y., Borisova, S.N., Seto, N.O., Lowary, T.L., Palcic, M.M., Evans, S.V. (2008) ABO(H) blood group A and B glycosyltransferases recognize substrate via specific conformational changes. Journal of Biological Chemistry 283; 10097-10108.
- Letts, J.A., Persson, M., Schuman, B., Borisova, S. N., Palcic, M.M., Evans, S.V. (2007) The effect of heavy atoms on the conformation of the active-site polypeptide loop in human ABO(H) blood-group glycosyltransferase B. Acta Crystallographica D Biological Crystallography. 63; 860-865.
- Hoseinni−Maaf, B., Letts, J.A., Persson, M., Smart, E., LePennec, P−Y., Hustinx, H., Zhao, Z., Palcic, M.M., Evans, S.V., Chester, M.A., Olsson, M.L. (2006) Structural basis for red cell phenotypic changes in newly−identified, naturally−occurring subgroup mutants of the human blood group B glycosyltransferase. Transfusion 47; 864-875.
- Persson, M., Letts, J.A., Hoseinni−Maaf, B., Borisova, S.N., Palcic, M.M., Evans, S.V., Olsson, M.L. (2006) Structural effects of naturally−occurring human blood group B galactosyltransferase mutations adjacent to the DXD motif. Journal of Biological Chemistry 282; 9564-9570.
- Letts, J. A., Rose, N. L., Fang, Y. R., Barry, C. H., Borisova, S. N., Seto, N. O. L., Palcic, M. M., Evans, S. V. (2006) Differential Recognition of the Type I and Type II H−antigen Acceptors by the Human ABO(H) Blood Group A and B Glycosyltransferases. Journal of Biological Chemistry 281; 3625-3632.